Background
Orelabrutinib is a novel, small molecule, selective irreversible Bruton tyrosine kinase inhibitor, approved in China for the treatment of patients with relapsed/refractory (R/R) chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL), mantle cell lymphoma (MCL), and marginal zone lymphoma (MZL). The previous publication of this study reported that orelabrutinib is highly active in R/R Waldenström's macroglobulinemia (WM), with a well-tolerated safety profile (EClinicalMedicine. 2022;52:101682). We present here the long-term results of this study.
Methods
This is a prospective, multicenter study of orelabrutinib in patients with WM who had at least one prior line of treatment (ClinicalTrials NO. : NCT04440059). Orelabrutinib was administered orally at a daily dose of 150 mg until disease progression or unacceptable toxicity. Genotyping using MYD88 L265P and CRCR4 S338X mutation were performed with qPCR assay. The primary endpoint was major response rate (MRR) assessed by the Independent Review Committee (IRC) according to IWWM-6 (Br J Haematol. 2013; 160: 171-176).
Results
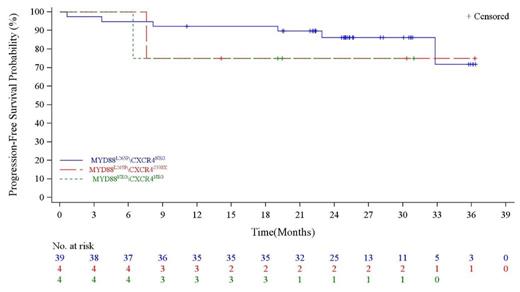
Between August 2019 and December 2020, 66 R/R WM patients were assessed for eligibility. Forty-seven eligible patients were evaluated for efficacy at a median follow-up of 31.9 months (interquartile range: 28.0,36.5). As assessed by IRC, the MRR was 80.9%, and the overall response rate was 91.5%. The PFS rates was estimated as 72.1% at 36 months and the median PFS has not been reached. There is no significant difference in PFS across various MYD88 L265P and CRCR4 S338X genotype (P=0.69; Figure 1). The 30-months PFS rate in MYD88 L265P/CXCR4 NEG, MYD88 L265P/CXCR4 S338X, and MYD88 NEG/CXCR4 NEG were 86.2%, 75% and 75%, respectively. Most adverse events were Grades 1 or 2 (63.8%). The common grade 3 or higher adverse events occurred were neutropenia (10.6%), thrombocytopenia (8.5%), and pneumonia (6.4%). Treatment related serious adverse events were reported in 8.5% patients. Adverse events leading to discontinuation occurred in 4 patients (8.5%). One treatment-related death was reported (hepatitis B reactivation).
Conclusions
Results with longer follow-up continue to show that orelabrutinib has high, deep and durable response in patients with R/R WM. Data support the safety of long-term orelabrutinib treatment in R/R WM, with no new safety signals identified.
OffLabel Disclosure:
No relevant conflicts of interest to declare.
Orelabrutinib is a newly developed BTK inhibitor with high selectivity. It is highly potent against BTK with notable less off-target inhibition of other tyrosine kinases.High kinase selectivity, persistent BTK target occupancy, potent anti-tumor activity, and the safety profile support orelabrutinib as an alternative treatment option for WM

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal